What is an atom?
An atom a fundamental piece of matter. (Matter is anything that can be touched physically.) Everything in the universe (except energy) is made of matter, and, so, everything in the universe is made of atoms.An atom itself is made up of three tiny kinds of particles called subatomic particles: protons, neutrons, and electrons. The protons and the neutrons make up the center of the atom called the nucleus and the electrons fly around above the nucleus in a small cloud. The electrons carry a negative charge and the protons carry a positive charge. In a normal (neutral) atom the number of protons and the number of electrons are equal. Often, but not always, the number of neutrons is the same, too.
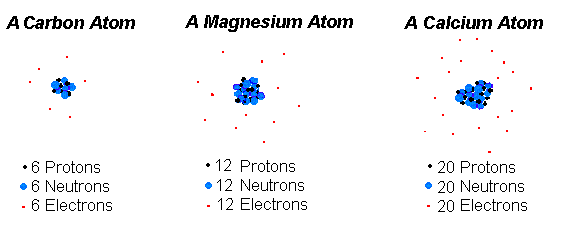
The negative electrons are attracted to the positive nucleus by the same electrical force which causes magnets to work. That's what keeps the atom together.
GLOSSARY
C
Calcium: Is an element of the Periodic Table of Chemical Elements, it symbol is Ca and it has 2, 8, 8, 2 electrons per shell.
E
Electron: Subatomic particle of the atom, has negative electrical charge and it is outside of the atom, surrounding it.
M
Magnesium: Is an element of the Periodic Table of Chemical Elements, it symbol is Mg and it has 2, 8, 2 electrons per shell.
N
Neutron: Subatomic particle of the atom, has neutral electrical charge and it is in the nucleus of the atom.
P
Proton: Subatomic particle of the atom, has positive electrical charge and it is in the nucleus of the atom.
S
Subatomic: that forms part of the atom, for example: the electron is a SUBATOMIC particle, because it´s a part of the atom.
No comments:
Post a Comment